eQMS
Guide for achieving rapid success with QMS
May 3, 2023How eQMS helps to avoid FDA Warning letters?
December 2, 2022Step-by-Step instructions to write a Non-Conformance Report
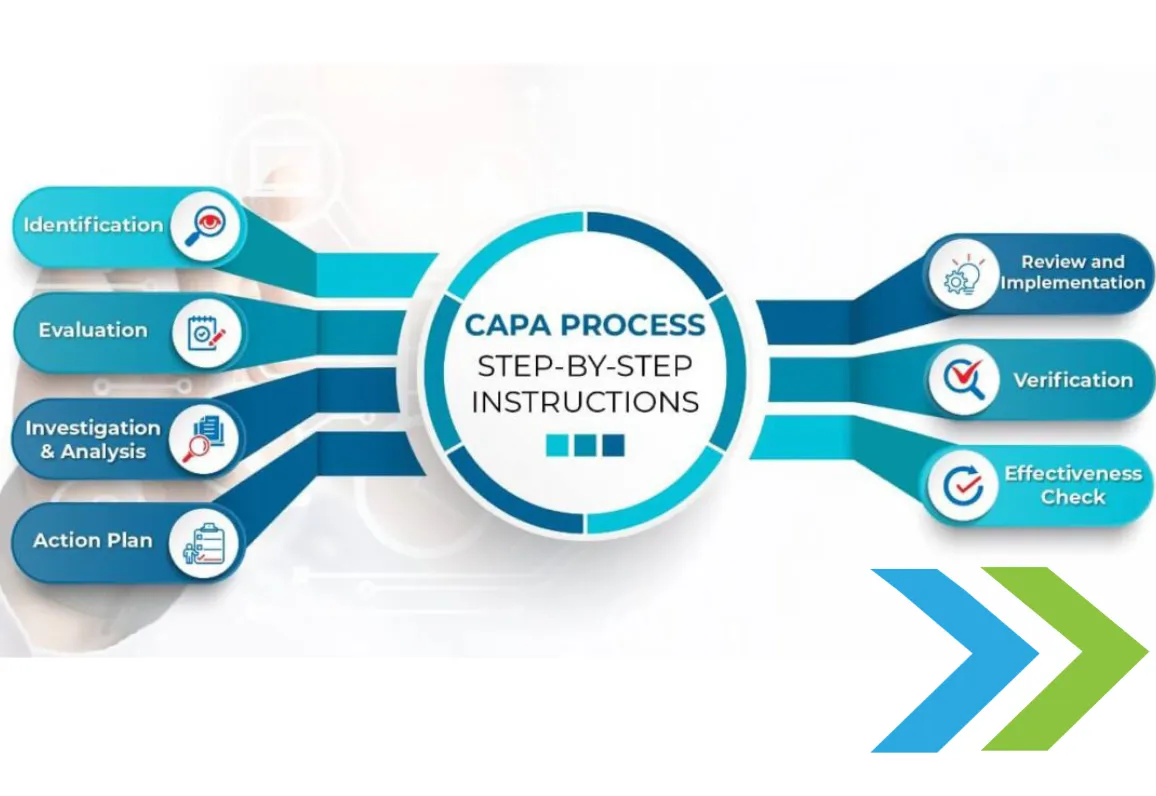
November 30, 2021CAPA PROCESS – STEP-BY-STEP INSTRUCTIONS
November 30, 2021Quality Assurance vs. Quality Control
November 2, 2021Why Non-Conformance Management is important?
October 21, 2021Your Quality Management is Failing and Here’s Why
September 30, 2021From Root Cause to Real Cause
September 2, 20217 Key Elements of a Quality Management Software
August 12, 2021FDA 483 Observations vs. Warning Letter
July 28, 2021CAPA Process Improvements
July 20, 2021Step by Step approach to achieve an FDA/MDIC approved Quality Management System
May 5, 2021
- 1
- 2