Guide for achieving rapid success with QMS

Article Context:
Bygones are those days when quality management systems (QMS) were largely confined to expensive, on-site platforms designed specifically for larger corporations. Nowadays, a plethora of adaptable cloud-based options are available, making QMS accessible to organizations of all sizes.
However, even with a wider range of paperless/electronic QMS solutions to select from, a smooth transition to a new system is not assured. The life sciences companies operating in strictly regulated sectors struggle with unsuccessful validation, adoption, and implementation journeys.
The reason is – many eQMS (electronic quality management system) providers face challenges in providing efficient onboarding experiences, resulting in their customers expending precious resources such as time and capital during this crucial mobilization stage. Compounding it – is the complexity and changing FDA 21 CFR Part 11 and GAMP computer system regulations.
Therefore, what actions should your life science organization take to optimize the worth of its eQMS investment during the validation, embracing, and implementation stages?
eQMS team has utilized their vast knowledge from working with regulated life science clients to equip you with all the insider insights necessary for a successful eQMS validation, adoption, and implementation journey.
Let us dig into the details.
Validating the QMS
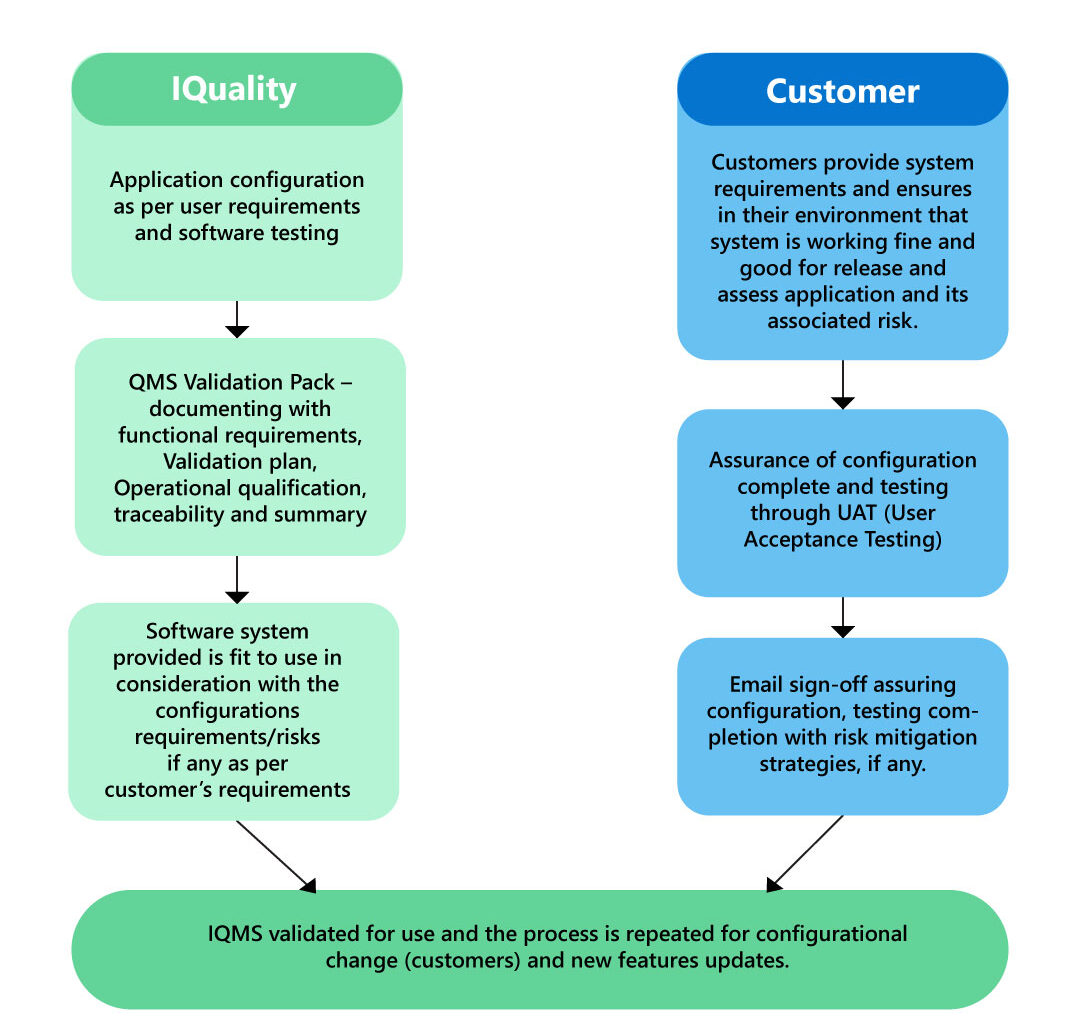
This is the initial and crucial step in embracing and adopting eQMS software. It entails verifying that the software conforms to FDA and other international regulatory requirements and standards.
To simplify the eQMS validation phase, all you have to do is to follow the below guidelines:
Vendor Audit
Follow the vendor’s software development and testing procedures to evaluate. By comprehending and establishing trust in the vendor’s practices, you can leverage the testing efforts that the vendor is already performing, rather than starting from scratch to recreate testing environments. Additionally, review any supporting documentation provided by the vendor to facilitate the process.
The Quality team supplies their clients with all the documentation required to complete eQMS software validation rapidly and easily, as well as receives and supports template content and guidance to ensure a seamless configuration and implementation streamlining their quality process and procedures.
Embracing the QMS
In this phase, as a new user, you must focus on a few areas within your new eQMS, including account management, configurations, document management, training management, and analytics (reports and analysis)
QMS Software streamlines and simplifies the eQMS adoption process. Below are the required steps you should follow:
Account creation and Onboarding
In most cases, as a basic user, you will be able to read and train on documents assigned to you. With additional access, you can author, review, and approve documents. Furthermore, you can complete training, track progress, read documents authored by others, and manage your account.
As a quality user, you have complete control. You’ll have full access to everything inside the software and the capability to manage all aspects of the system, including:
Configurations: Role-based access controls, process-template management, training requisition settings, and protocols.
Document management: Create, approve, publish, and retire documents.
Training management: Create, manage, and monitor training programs and analyze training progress.
Reports: Track and monitor access and analyze user activities and quality processes.
Besides gaining a thorough understanding of essential eQMS features, training aligns your business and encourages everyone to ask questions and suggest application ideas.
Implementation
The implementation phase is a critical step toward achieving success with your eQMS. Here are some steps you can take to ensure a streamlined implementation process:
Clearly define your implementation goals, timeline, and user adoption rates. Establish a plan for dealing with any deviations that may occur during the implementation process.
Involve stakeholders from various departments to ensure a comprehensive and effective implementation. Ensure that everyone has received appropriate training to maintain a quality-first mindset.
Stay up to date with system updates that may impact your validated state. Implement change management protocols to ensure compliance with regulations and standards.
Don’t hesitate to seek assistance from the dedicated customer success teams. At Compliance Group, we offer an industry-leading implementation pathway that can be completed in as little as 60 days.
Our end-to-end digital management system includes dashboards, document management, training, reports, quality events, and change management. We also provide best-in-class flexibility and automation to help you build, track, and update your processes quickly and easily.
To get more information, contact our sales – support team for a free demo. Write to us at sales@complianceg.com